Non-Radioactive Protein Combination Naming: A More secure and Effective Methodology
Protein combination naming is a basic procedure utilized in sub-atomic science and natural chemistry to concentrate on protein articulation, connections, and changes. Customarily, radioactive isotopes, for example, sulfur-35 or tritium were utilized to name proteins. While powerful, these radioactive techniques present critical security perils, require particular offices, and create risky waste. **Non-radioactive protein combination labeling has arisen as an imaginative other option, giving a more secure, more manageable, and similarly compelling way to deal with concentrating on proteins. This article investigates
Understanding Non-Radioactive Protein Combination Marking
Non-radioactive protein amalgamation naming alludes to strategies that include labeling proteins with non-radioactive markers or tests. These markers are integrated during or after protein blend and permit scientists to follow, evaluate, and examine proteins without utilizing destructive radioactive isotopes.
The non-radioactive methodology is especially significant in different fields, including drug disclosure, proteomics, and demonstrative exploration. It uses progressions in fluorescent colors, biotin marking, and stable isotopes like nitrogen-15 or carbon-13. These options empower exact protein following while at the same time wiping out gambles related with radioactive materials’ the standards, strategies, and benefits of non-radioactive naming in protein combination.
Common Methods for Non-Radioactive Protein Synthesis Labeling
Several methods are widely used in non-radioactive protein labeling, each offering unique benefits depending on the research goals:
Fluorescent Labeling
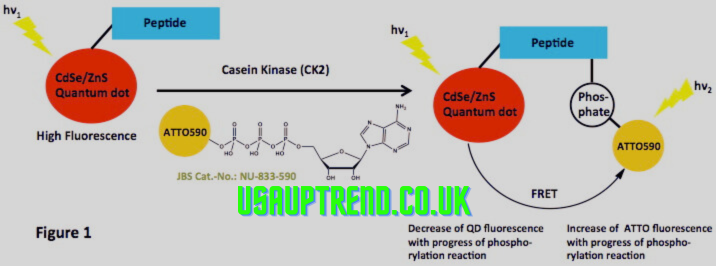
Fluorescent colors, for example, Alexa Fluor, Cy colors, and fluorescein, are among the most famous apparatuses in non-radioactive protein blend marking. These colors tie to amino corrosive buildups in proteins, radiating light when energized by unambiguous frequencies. This strategy is exceptionally touchy and is great for live-cell imaging, protein limitation studies, and protein connection investigation.
Biotin Labeling
Biotin, a little particle, is much of the time utilized for non-radioactive naming because of its high proclivity for streptavidin. Proteins can be labeled with biotin either during union or post-translationally. The biotin-streptavidin framework is broadly utilized in Western smudging, ELISA, and pull-down tests due to its heartiness and similarity with different discovery strategies.
Advantages of Non-Radioactive Protein Synthesis Labeling
Switching to non-radioactive methods offers several significant advantages, making it a preferred choice in modern research laboratories
Enhanced Safety
Non-radioactive protein union marking disposes of the risks related with taking care of radioactive materials. Analysts are not generally presented to hurtful radiation, and research facilities don’t require specific safeguarding or garbage removal conventions. This makes non-radioactive techniques more secure for both the climate and specialists.
Sustainability
Radioactive garbage removal is costly and earth harming. Non-radioactive techniques limit ecological effect by lessening unsafe waste, lining up with worldwide maintainability objectives.
High Responsiveness and Resolution
Procedures, for example, fluorescent marking and stable isotope joining give magnificent awareness and goal, empowering the discovery of proteins at exceptionally low focuses. High level imaging and mass spectrometry strategies further improve the precision of these techniques.
Versatility
Non-radioactive protein blend naming techniques are exceptionally flexible. They can be adjusted to different applications, including live-cell imaging, in vitro examines, and high-throughput proteomics. This adaptability makes them reasonable for an extensive variety of exploration fields.
Regulatory Compliance
Using non-radioactive methods simplifies compliance with regulatory standards. Laboratories do not need to obtain radioactive material licenses or adhere to stringent disposal regulations, reducing administrative and operational burdens.
Applications of Non-Radioactive Protein Synthesis Labeling
Non-radioactive labeling techniques have revolutionized research in several areas:
- Drug Development: Fluorescently named proteins assist with screening drug competitors by imagining associations among proteins and medication particles.
- Proteomics: Stable isotope naming empowers the quantitative examination of protein articulation and post-translational adjustments in complex organic examples.
- Diagnostics: Biotin-streptavidin marking frameworks are normally utilized in demonstrative examines for distinguishing explicit biomarkers in illnesses like malignant growth or immune system problems.
- Cell Biology: Fluorescent colors consider ongoing imaging of protein elements in live cells, giving experiences into cell processes.
Difficulties and Future Points of view
In spite of its many benefits, non-radioactive protein blend naming isn’t without challenges. For example, a few techniques require costly reagents or modern hardware, which might restrict their openness to more modest research facilities. Furthermore, consolidating non-radioactive marks can once in a while modify protein capability or construction, possibly influencing the precision of results.
Be that as it may, progressing headways in innovation are tending to these constraints. Developments in color science, isotopic marking, and chemical based strategies are making non-radioactive protein union naming more reasonable, productive, and dependable.
Later on, the combination of man-made reasoning (computer based intelligence) and AI into proteomics could additionally upgrade the capacities of non-radioactive naming.
These technologies can analyze vast datasets generated by techniques like SILAC, enabling deeper insights into protein functions and interactions.
FAQ
What distinguishes radioactive protein labeling from non-radioactive protein labeling?
While non-radioactive labeling uses chemical or enzymatic markers that do not provide a radiation danger, radioactive labeling uses isotopes that produce radiation for detection.
Which technique works best for tracking proteins in vivo?
Because non-radioactive methods are safe and compatible with living cells, they are favored, particularly enzymatic labeling and click chemistry.
In comparison to conventional methods, how accurate are non-radioactive methods?
High sensitivity and precision are provided by contemporary non-radioactive techniques, which are frequently on par with radioactive markers.
Are there commercially accessible non-radioactive labeling kits?
Indeed, a lot of commercial kits come with ready-to-use chemicals and procedures for a variety of labeling methods.
Which safety procedures apply to labeling experiments that aren’t radioactive?
Without taking extra measures against radiation, standard biosafety recommendations that concentrate on handling chemicals and disposing of waste are applicable.
Conclusion
Non-radioactive protein synthesis labeling represents a significant leap forward in molecular biology research. By supplanting unsafe radioactive strategies with more secure and more reasonable other options, analysts can accomplish high responsiveness and accuracy in protein examination. From fluorescent colors to stable isotopes, the flexibility of non-radioactive strategies has made them crucial in fields like medication revelation, proteomics, and diagnostics.
As innovation keeps on developing, non-radioactive naming techniques will without a doubt turn out to be much more open and strong, preparing for earth shattering revelations in life sciences. This imaginative methodology shields analysts and the climate as well as upgrades the degree and profundity of logical investigation
Also read more: Technologies Hearthssgaming