Cell signaling technology KRAS focuses on how the KRAS gene regulates cell communication and signaling pathways. KRAS is a key protein controlling cell growth, division, and survival. Mutations in this gene often cause uncontrolled proliferation of cells, which in most cases is associated with cancer. Why is knowing KRAS important in the study of cancer? Usually, the mutations in the gene help promote tumor growth. Knowing how these mutations work leads to more potent therapies for several cancers.
Scientists are also continuing to research in an effort to understand how mutations of the KRAS gene work. Targeting such mutations with therapy is the prime direction toward the prevention and slow progression of cancer to significantly improve patient outcomes.

What is Cell Signaling Technology KRAS?
KRAS signaling is basic to transducing signals from the cell surface receptors to the nucleus, which influences a host of key processes such as cell growth, differentiation, and survival. KRAS is a small GTPase; it is an enzyme type that acts as a molecular switch, cycling between its active GTP-bound and inactive GDP-bound states. Therefore, an active KRAS molecule initiates downstream signaling cascades, such as the MAPK/ERK and PI3K/AKT pathways, critical for normal cellular function.
Therefore, an active KRAS molecule initiates downstream signaling cascades, such as the MAPK/ERK and PI3K/AKT pathways, critical for normal cellular function.
However, mutations in the KRAS gene can permanently activate it independent of signals from outside. It is this that maintains the downstream pathways in a state of permanent stimulation and hence continues to induce cell division uncontrolled, which is linked with the development of tumors. Since KRAS signaling has direct associations with cancers like pancreatic, lung, and colorectal cancer, this is highly relevant in the research on cancer. The delineation of the molecular mechanisms of KRAS activation and its impact on cell signaling pathways has thus led to new targets in developing specific therapies against cancer.
What Does Cell Signaling Technology Do?
Cell signaling technology provides the tools and techniques to understand how cells communicate and respond to signals in their environment. It allows researchers to probe the complexity of networks regulating cell processes through signaling molecules. Regarding KRAS, cell signaling technology helps the scientist understand how mutations within that gene lead to changes in normal pathways, contributing to cancer.
Cell signaling technology is one of the valuable technologies that may actually point at specific points in a signalling pathway where therapeutic intervention can be effective. For instance, techniques like Western blotting, immunoprecipitation, and mass spectrometry now allow investigators to detect and quantify the involvement of proteins in KRAS signaling. In addition, the utilization of CRISPR-Cas9 gene editing and RNA interference (RNAi) technologies allows the manipulation of KRAS and other signalling proteins to study them more in detail. These break-throughs are helping reveal new drug targets and formulate therapies that specifically inhibit aberrant signaling caused by the mutations in KRAS.
What is the molecular weight of KRAS?
The molecular weight of KRAS is about 21 kilodaltons. This is a small GTPase protein, and it comes from the KRAS gene situated in chromosome 12 within the human species. As small as it is, KRAS protein performs an essential function in cellular signaling. Molecular weight is the fundamental characteristic of KRAS because that is what dictates its functions, how it interacts with other proteins, and what area it will localize at.
KRAS comprises 188 amino acids, while the atomic masses of amino acids provide the molecular weight for KRAS. Also, detection and analysis processes involving experimental studies would significantly depend on the molecular weight of KRAS. It will therefore determine whether the process, during the study conducted with western blotting or examination through mutation status to find an expression of the gene is valid. The accuracy in the identification and measurement of KRAS would be critically important for determining its role in cancer as well as for developing therapy that targets its function.
How Big Is the RAS Protein?
KRAS, HRAS, and NRAS are a family of RAS proteins of around 21 kDa in size or their molecular weight. Being a small GTPase is because it binds both GTP and GDP for regulation of its activity due to the small size of the RAS proteins. Such a small-sized protein molecule may act as molecular switches in the cell and it does cycle quickly and rapidly from an active state to the inactive state upon receipt of many different kinds of signals.
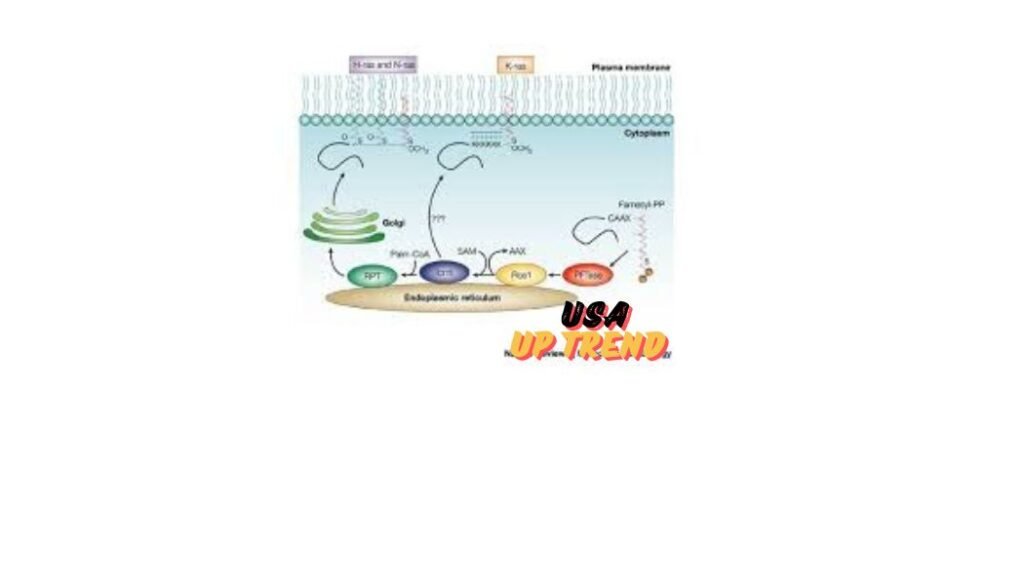
Localizing RAS proteins at the inner leaflet of the plasma membrane positions them on the cell surface to interact with many effectors that will culminate in cellular signals. Even though these are small molecules, their activities do determine significant roles in affecting cell signaling pathways related to growth, differentiation, and death. Mutations of the RAS protein, in particular KRAS, fuel aberrant signaling that results in the promotion and perpetuation of cancer. Understanding the structural and functional nature of RAS proteins could be crucial in the development of effective therapies that can impair their activity in cancerous cells.
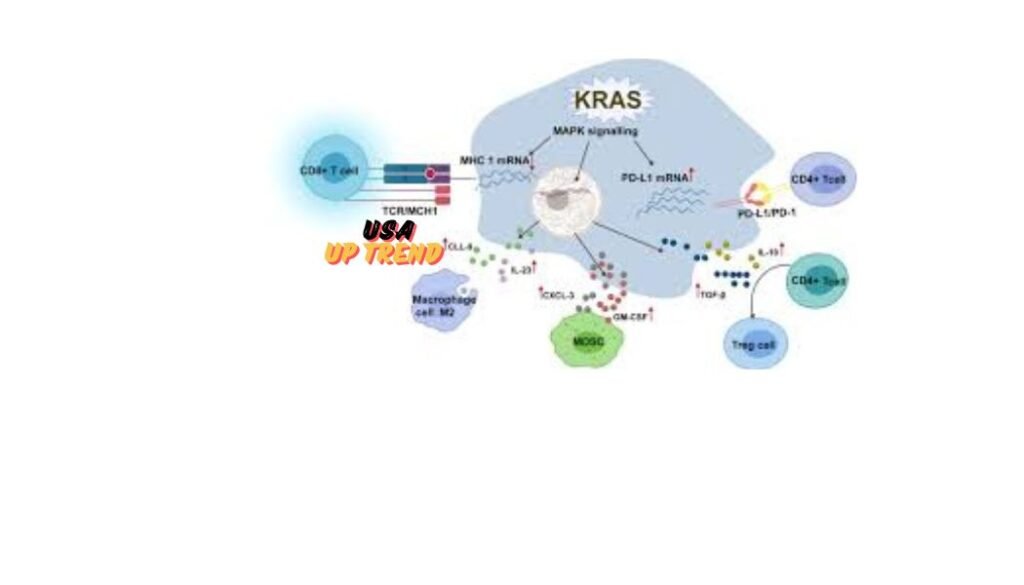
KRAS Mutations Role in Cancer
KRAS mutations make up most of the most frequently identified genetic changes in human cancer. These mutations occur more commonly at certain codons, such as codons 12, 13, and 61 of the KRAS gene regulating its GTPase activity, and the most common form of mutation is the replacement of glycine with aspartic acid at position 12 (G12D).
which traps the protein in its active state-bound GTP, requiring this protein to maintain signals on continuously and drive cell divisions outside of regulatory mechanisms that keep cell growth in a tightly controlled manner.
KRAS mutations make up most of the most frequently identified genetic changes in human cancer. These mutations occur more commonly at certain codons, such as codons 12, 13, and 61 of the KRAS gene regulating its GTPase activity, and the most common form of mutation is the replacement of glycine with aspartic acid at position 12 (G12D), which traps the protein in its active state-bound GTP, requiring this protein to maintain signals on continuously and drive cell divisions outside of regulatory mechanisms that keep cell growth in a tightly controlled manner.
This mutation eliminates the regulation of normal mechanisms of cellular proliferation and apoptosis, thereby permitting unrestricted growth, anti-apoptosis, and metastasizing ability in cancerous cells. KRAS is the most common mutated oncogene in pancreatic cancer; in fact, over 90% of pancreatic cancer patients carry this mutation. Similarly, KRAS mutations are often linked with lung adenocarcinoma and colorectal cancer thus remain a prime area for the study of cancer pathogenesis and drug development.
Cell Signaling Technology in the Targeting of KRAS
The challenge that researchers face while targeting KRAS is that it is of a very small size and also has no well-defined, pitched binding pockets on its surface. However, with new technologies regarding cell signaling recently, researchers have been able to produce a small molecule inhibitor which targets certain specific mutant forms of KRAS. This can be reflected by a breakthrough such as G12C mutant form inhibitors, targeting KRAS most often found in lung cancer.
Structure-based drug design and high-throughput screening technologies have significantly contributed to the identification of these inhibitors. The knowledge of the structural biology of KRAS and its interaction with other signaling molecules has guided the researchers in designing drugs that specifically bind to the mutant KRAS proteins and block their activity. These inhibitors are now showing promise in clinical trials and give new hope to patients whose cancers are drive by mutations in KRAS.
Problems in the Identification of KRAS Inhibitors
Although significant advances have been make in the area, there are still substantial barriers to developing effective therapies aim at KRAS. In particular, the high redundancy of signaling pathways downstream of KRAS is one such barrier. Even when KRAS is inhibited successfully, cancer cells can bypass the block by activating alternative pathways that allow them to proliferate.
Furthermore, the non-reactive surface and the low molecular weight of KRAS make it challenging to design drugs that can very highly specifically and selectively bind with the protein. All early attempts at targeting KRAS had failed because the protein had no accessible surface binding sites. That scenario has now changed with the discovery of covalent inhibitors that irreversibly bind to mutant KRAS, thus opening up routes to target this hard-to-target protein.
KRAS Mutations and Associated Cancers
| KRAS Mutation | Associated Cancer |
| G12D | Pancreatic cancer |
| G12C | Lung adenocarcinoma |
| G13D | Colorectal cancer |
| Q61H | Thyroid cancer |
| Signaling Pathway | Function |
| MAPK/ERK | Cell proliferation |
| PI3K/AKT | Cell survival |
| RAL-GEF | Vesicle trafficking |
| RAF-MEK-ERK | Differentiation |
Future Directions in KRAS Research
Further studies into KRAS and its roles in cancer have developed new approaches to the target this protein. Breeding hopes for using combination therapies that simultaneously inhibit multiple pathways, blocking KRAS and its downstream effectors will help to overcome redundancy and resistance mechanisms that have limited utility for single-agent therapies.
Another area of focus is the development of immunotherapies targeting KRAS-mutant cancers. Recent studies have indicated that some KRAS mutations may even create neoantigens—new proteins recognized by the immune system as foreign. The power of the immune system to recognize and destroy KRAS-mutant cells has led to the exploration of new avenues in the treatment of cancers resistant to conventional therapies.
Conclusion
Such key cell processes as growth, differentiation, and survival can be regulate by one of the major cell signaling regulators, and this includes KRAS. This leads to the reason that numerous aggressive types of cancer are associate with mutations of the KRAS gene; this is a point of considerable interest in conducting relevant research. The advancements in cell signaling technologies revealed the fact that these mutations direct a pathway of cancer development and their work has begun in developing some targeted therapies towards the inhibition of KRAS-driven tumor growth. Of course, it is rather tough to target KRAS for its structure as well as for pathway redundancy, but there is new hope by adopting strategies like small molecule inhibitors and combination treatment in looking toward more effectiveness in the coming years for designing therapies in the case of cancer.
FAQs
What is KRAS and why is its role so crucial in this domain of cancer research?
KRAS is a protein that encodes for a gene involved in cell growth and the division process. Mutations resulting in KRAS are found in most cancers, but KRAS is one of the least understood genes in cancer research relating to treatment; therefore, cell signaling technology enters the scene.
How does cell signalling technology aid in the investigation of KRAS?
Cell signaling technology helps researchers explore how KRAS mutations have changed normal cell signaling. It, therefore, establishes new targets for treatment and develop treatment methods for KRAS-induced cancers.
Which of the cancers relate to KRAS mutations?
KRAS mutations are commonly linked to pancreatic, lung, or colorectal cancers. Normally, these mutations cause unruly growth of cells as they are often harder to treat.
Why would KRAS mutations be particularly hard to target with the regular treatments?
KRAS mutations create proteins that are hard to target for inhibition due to their shape and function. New drugs are coming in the form of small molecule inhibitors that can specifically target these mutations.
What are pathways of cell signaling regulated by KRAS?
KRAS mediates pathways involved in regulating cell growth, survival, and differentiation. Mutated KRAS leads to unchecked overactivation of the pathways leading to aberrant cell growth and cancer.
Can KRAS mutations be detected early?
Yes, the diagnostic tools advance so that KRAS mutations can be diagnosed early, and therefore, there can be planning in treatment, and results will thus improve.
What future developments might researchers expect in KRAS cancer research?
Possible future developments include better KRAS inhibitors, combination therapies, and personalized medicine approaches to target specific KRAS mutations for better cancer treatment.
Read more about Technology at USA UP TREND




